Certified HIPAA Professional Exam Braindumps
Killexams.com HIO-201 Exam Braindumps contain complete question pool, updated in April 2024 including VCE exam simulator that will help you get high marks in the exam. All these HIO-201 exam questions are verified by killexams certified professionals and backed by 100% money back guarantee.
HIO-201 thinking - Certified HIPAA Professional Updated: 2024 | ||||||||||||||||||||||
| Remember these HIO-201 dumps and enroll for the test | ||||||||||||||||||||||
 |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
Exam Code: HIO-201 Certified HIPAA Professional thinking January 2024 by Killexams.com team | ||||||||||||||||||||||
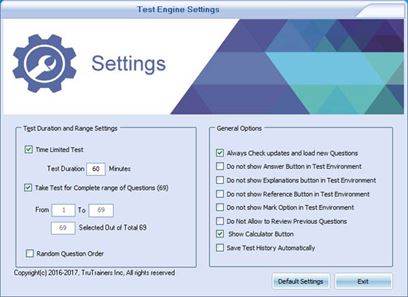
HIO-201 Certified HIPAA Professional Exam: HIO-201 (Certified HIPAA Professional) Exam Details: - Number of Questions: The exam consists of multiple-choice questions. - Time: Candidates are typically given a specified amount of time to complete the exam. Course Outline: The Certified HIPAA Professional (CHP) course is designed to provide candidates with a comprehensive understanding of the Health Insurance Portability and Accountability Act (HIPAA) regulations and their implications in healthcare organizations. The course outline includes the following topics: 1. Introduction to HIPAA - Overview of HIPAA regulations - HIPAA Privacy Rule and Security Rule - HIPAA enforcement and penalties 2. HIPAA Privacy Rule - Protected health information (PHI) - Patient rights and consent - Privacy practices and policies 3. HIPAA Security Rule - Security safeguards and requirements - Risk assessment and management - Security policies and procedures 4. HIPAA Transactions and Code Sets - Electronic data interchange (EDI) - Standard transactions and code sets - Compliance requirements 5. HIPAA Enforcement and Compliance - HIPAA audit and compliance programs - Breach notification and reporting - Business associate agreements Exam Objectives: The HIO-201 exam aims to assess candidates' knowledge and understanding of HIPAA regulations and their practical application in healthcare settings. The exam objectives include: 1. Demonstrating knowledge of HIPAA regulations, including the Privacy Rule and Security Rule. 2. Understanding the requirements for safeguarding protected health information (PHI). 3. Applying security measures and risk management principles to ensure HIPAA compliance. 4. Understanding HIPAA transactions and code sets for electronic data interchange. 5. Familiarity with HIPAA enforcement and compliance programs, including breach notification and business associate agreements. Exam Syllabus: The exam syllabus covers the following topics: - Introduction to HIPAA - HIPAA Privacy Rule - HIPAA Security Rule - HIPAA Transactions and Code Sets - HIPAA Enforcement and Compliance Candidates are expected to have a solid understanding of these Topics and demonstrate their ability to apply HIPAA regulations in real-world scenarios. The exam assesses their knowledge, comprehension, and proficiency in various aspects of HIPAA compliance. | ||||||||||||||||||||||
| Certified HIPAA Professional HIPAA Professional thinking | ||||||||||||||||||||||
Other HIPAA examsHIO-201 Certified HIPAA ProfessionalHIO-301 Certified HIPAA Security | ||||||||||||||||||||||
| Simply experience our HIO-201 Questions bank and feel certain about the HIO-201 test. You will pass your HIO-201 exam at Full Marks or your cash back. All that you have to pass the HIO-201 exam is given here. We have accumulated a database of HIO-201 Dumps taken from real exams in order to allow you to prepare and pass HIO-201 exam on the simple first attempt. Essentially set up our VCE HIO-201 exam Simulator and practice. You will pass the exam. | ||||||||||||||||||||||
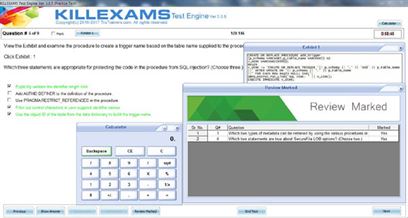
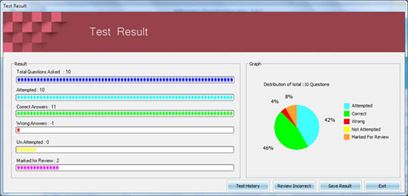
| HIPAA HIO-201 Certified HIPAA Professional https://killexams.com/pass4sure/exam-detail/HIO-201 HIO-201 Question: 169 Periodic testing and revision of contingency plans is addressed by: A. Testing and Revision Procedures B. Information System Activity Review C. Response and Reporting D. Data Backup Plan E. Emergency Access Procedure Answer: A Question: 170 Select the FALSE statement regarding the administrative requirements of the HIPAA privacy rule. A. A covered entity must mitigate, to the extent practicable, any harmful effect that it becomes aware of from the use or disclosure of PHI in violation of its policies and procedures or HIPAA regulations. B. A covered must not in any way intimidate, retaliate, or discriminate against any individual or other entity, which files a complaint. C. A covered entity may not require individuals to waive their rights as a condition for treatment, payment, enrollment in a health plan, or eligibility for benefits. D. A covered entity must retain the documents required by the regulations for a period of six years. E. A covered entity must change its policies and procedures to comply with HIPAA regulations no later than three years after the change in law. Answer: E Question: 171 One implementation specification of a contingency plan is: A. Risk analysis B. Applications and Data Criticality Analysis C. Risk Management D. integrity Controls 60 HIO-201 E. Encryption Answer: B Question: 172 One implementation specification of the Security Management Process is: A. Risk Analysis B. Authorization and/or Supervision C. Termination Procedures D. Contingency Operations E. Encryption and Decryption Answer: A Question: 173 Maintenance personnel that normally have no access to PHI are called in to investigate water that is leaking from the ceiling of the room where a large amount of PHI is stored. The room is normally secured but the file cabinets have no doors or locks. Situations like this are addressed by which Workforce Security implementation specification? A. Risk Management B. Written Contract or Other Arrangement C. Accountability D. Authorization and/or Supervision E. integrity Controls Answer: D Question: 174 Which transaction covers information specific to accidents? A. Accident Report. B. First Report of Injury. C. Health Care Claim. D. Health Care Claim Payment/Advice. 61 HIO-201 E. Premium Payment. Answer: B Question: 175 The Health Care Claim Status Response (277) can be used in a number of ways. Select the correct usage. A. As a response to a health care claim status request B. As a health care claim payment advice C. Electronic funds transfer D. As a request for health care claims status E. Request for the psychotherapy notes of a patient Answer: A Question: 176 Select the best example of a business associate (if they had access to PHI). A. Accountants B. Hospital employees C. A covered entity’s internal IT department D. CEO of the covered entity E. The covered entity’s billing service department Answer: A Question: 177 The objective of this document is to safeguard the premises and building from unauthorized physical access and to safeguard the equipment therein from unauthorized physical access, tampering and theft A. Contingency Plan B. Facility Security Plan C. Emergency Mode Operation Plan D. Accountability E. Device and Media Controls 62 HIO-201 Answer: B Question: 178 The Integrity security standard has one addressable implementation standard which is: A. Encryption B. Authorization and/or Supervision C. Mechanism to Authenticate Electronic PHI D. Applications and Data Criticality Analysis E. Isolating Health care Clearing House Functions Answer: C Question: 179 This HIPAA security area addresses the use of locks, keys and procedures used to control access to computer systems: A. Administrative Safeguards B. Physical Safeguards C. Technical Safeguards D. Audit Controls E. Information Access Management Answer: B Question: 180 The transaction number assigned to the Health Care Eligibility Request transaction is: A. 270 B. 276 C. 278 D. 271 E. 834 63 HIO-201 Answer: A 64 For More exams visit https://killexams.com/vendors-exam-list Kill your exam at First Attempt....Guaranteed! | ||||||||||||||||||||||
|
3rd Party Risk Management , Application Security , Business Continuity Management / Disaster Recovery Attacks Exposed Millions of Records, Severely Disrupted Care and More
Hacks on healthcare sector entities reached record levels in 2023 in terms of data breaches. But the impact of hacks on hospital chains, doctors' offices and other medical providers - or their critical vendors - goes much deeper than the exposure of millions of health records. See Also: JavaScript and Blockchain: Technologies You Can't Ignore In many cases, the hacks triggered other types of serious fallout - most notably disruption to patient care delivery - as well as other consequences, including financial and reputational damage. Plus, nearly all major data breaches these days end up in court. And the bigger the hack, the more proposed class action lawsuits that get filed. The Department of Health and Human Services' Office for Civil Rights' HIPAA Breach Reporting Tool paints a picture of the immense mountain of patient records exposed by hacks in 2023. The biggest of those incidents include a mix of entities - large healthcare delivery organizations, health plans, a pharmaceutical services company and a medical transcription firm. Of the 653 major health data breaches posted to the HHS OCR website in 2023 as of Dec. 15, affecting more than 116.5 million individuals, 515 were reported as hacking incidents, affecting nearly 108 million individuals. So while hacking incidents made up about 80% of those 2023 breaches, they accounted for nearly 93% of people whose information was compromised in major health data breaches reported to federal regulators so far this year. 10 Largest Health Data Breaches in 2023 Involving Hacks
Ransomware attacks by cybercriminals involving data exfiltration incidents soared in the healthcare sector in 2023. They included hacks on third-party software such as Progress Software' MOVEit and Fortra's GoAnywhere file transfer applications, resulting in thousands of victim entities globally across all sectors - and tens of millions of individuals - including in healthcare - having their information stolen. As of Dec. 15, the largest MOVEit incident affecting the healthcare sector appears to have been reported by Welltok, a medical patient communication services provider that is part of Virgin Pulse. Welltok's MOVEit breach has affected nearly 8.5 million individuals so far. Another latest large healthcare sector breach involving the MOVEIt file transfer software vulnerability exploit was reported to Maine's attorney general on Dec. 14 by Delta Dental. The dental insurance provider said it is notifying nearly 7 million individuals that their PHI was affected by a June 1 hack on the MOVEit software used by Delta. As of Dec. 15, the Delta Dental MOVEit hack had not yet been posted on the HHS OCR website of major HIPAA breaches. But once it is added to the federal tally, it will undoubtedly land among the top 10 largest health data breaches in 2023. The largest of all the MOVEit hacks so far across all industries - affecting 11 million individuals - was reported by U.S. government contractor Maximus, according to security firm Emsisoft. The Maximus hack compromised the protected health information of about 2.8 million people and counting, according to the company's breach report filed to HHS on Aug. 4. The Maximus hack also ranks among the top 10 largest health data breaches reported in 2023. Specialty HacksHacks on specialty services providers to the healthcare sector have also affected long lists of healthcare entity clients. "The data breach at medical transcription company Perry Johnson & Associates was a particularly noteworthy incident in the healthcare sector," said Ani Chaudhuri, co-founder and CEO of security firm Dasera. The breach affected nearly 9 million patients nationwide, including more than 4 million New Yorkers who are patients of Northwell Health and Crouse Health. "The PJ&A consequences were significant due to the potential for widespread identity theft," Chaudhuri said. "Such incidents emphasize the vulnerability of healthcare institutions, even those not typically in the limelight, underlining the need for comprehensive cybersecurity strategies regardless of the organization's size." Hacks on other specialty healthcare providers also affected large swaths of their patients. Those include a latest hacking breach reported by New York-based East River Medical Imaging that affected nearly 606,000 individuals. "Specialty healthcare providers are viewed as low-hanging fruit, based on their inability to employ effective preventive controls such as user training and vulnerability management, as well as a lack of monitoring the network for signs that prevention has failed in order to mitigate the impact with a rapid response," said Mike Hamilton, CISO and co-founder of security firm Critical Insight. "Combined with the fact that the records held are just as valuable as those in larger institutions, smaller specialty providers are easy and lucrative targets." While some cybercriminal groups appear to be skipping data encryption in their ransomware attacks and going straight to data exfiltration, many of the incidents involving data encryption have had a dramatic impact on their victims. These include attacks on hospital chains such as Prospect Medical and Ardent Health Services, which disrupted patient delivery services for weeks. But, of course, this isn't just an American problem. A ransomware attack in October on TransForm Shared Service Organization was still affecting its five hospital members in Ontario in December (see: Ontario Hospitals Expect Monthlong Ransomware Recovery). In that and many similar situations involving ransomware attacks on hospitals, patient treatments had to be postponed, canceled or shifted to other facilities that were not involved in the incident. "There have been numerous reports and studies documenting how radiation and other crucial cancer treatments have been canceled or delayed because of cyberattacks," Jon Moore, chief risk officer at privacy and security consultancy Clearwater, said. "We should not underestimate the profound impact this has on the well-being of these vulnerable patients." Similar attacks in Europe disrupted healthcare during the year. A July cyberattack against Swedish software and services vendor Ortivus severed access to digital health records for at least two National Health Service ambulance services in the United Kingdom. Paramedics had to resort to using pen and paper to manage patient information (see: Software Vendor Attack Slows Down 2 UK Ambulance Services). Though rare, the lasting impact of ransomware attacks pushed some already financially strapped healthcare entities over the edge - or pretty close - in 2023. In June, rural Illinois medical system St. Margaret's Health shut down permanently partly due to fallout from a 2021 ransomware incident. Meanwhile, the planned sale of Prospect Medical's Connecticut Health Systems - including three hospitals - to Yale New Haven Health is still in jeopardy due to worsening financial and other problems at the facilities in the aftermath of an August cyberattack on Prospect, according to local media outlet CT Mirror (see: Some Prospect Medical Hospitals in Dire State, Post-Attack). Prospect Medical, which is based in California and operates 17 hospitals, reported the incident to federal regulators as a health data breach affecting about 342,400 individuals. So while that attack didn't affect millions of patient records, as many other attacks did, the impact was just as significant for financial reasons. A handful of hacks on healthcare entities have been especially sinister. One that stands out in particular was the cyber assault on Lehigh Valley Health Network, Moore said. In that hack, BlackCat cybercriminals, frustrated by the lack of ransom payment from the health network, resorted to releasing sensitive medical photos of breast cancer treatment patients they had stolen from the health system (see: BlackCat Leaking Patient Data and Photos Stolen in Attack). "Beyond the breach itself, this deplorable act raises questions about the ethical boundaries of cybercriminals and the emotional toll it exacts on the affected patients and their families," Moore said. "As professionals in the healthcare industry, we sometimes become numb to the statistics and numbers associated with breaches and compromised records. However, these stories serve as poignant reminders of why the work we do is not just meaningful but vital," he said. "We must prioritize cybersecurity efforts not only to protect data but also to safeguard the lives and well-being of the patients and communities we serve. These personal stories are a testament to the significance and urgency of our mission in securing healthcare data and systems." Info stealer infections affecting employees' personal and enterprise devices exploded in 2023, said Scott Small, director of cyberthreat intelligence at security firm Tidal Cyber. In an attack in September on a French hospital in the city of Brest, he said, "Actors linked to the FIN12 group used valid credentials belonging to a healthcare professional to connect to an internet-exposed remote desktop service and gain backdoor access to the center's network. The credentials were likely compromised via info-stealing malware." "FIN12 was responsible for multiple high-profile ransomware attacks on U.S. hospitals in 2020 but has widened its targeting since," Small said. He added that the September attack demonstrates that healthcare remains a viable target for highly capable financially motivated actors including LockBit, Alphv/BlackCat and others. Wishful ThinkingA small share of noteworthy attacks in 2023 appear to not have had the impact some hacker groups might have wished for. They include a campaign early in 2023 in which the KillNet hacktivist group launched daily DDoS attacks against nearly 100 healthcare organizations, including pharmaceutical companies, hospitals and insurers (see: HHS, AHA Warn of Surge in Russian DDoS Attacks on Hospitals). "KillNet is one of several actor collectives behind coordinated denial-of-service attacks in latest months amid escalating conflict in the Middle East. These groups' heightened activity levels and known historical impact on the healthcare sector means they should remain on defenders' radars for the foreseeable future," Small said. Early warnings about the DDoS campaign from U.S. authorities and industry groups, including the American Hospital Association, helped to blunt the effect of the attacks on most hospitals (see: Cyber Fail: More Bumbling Cybercrooks, Avoidable Breaches). Looking AheadIn 2024, the healthcare sector needs to keep a watchful eye on other disturbing developments that started this year and will undoubtedly continue next year, some experts said. There has been a notable surge in business email compromise attacks, some of which creatively extended to text messaging and voicemail, according to Moore. While these incidents often escaped public attention due to a lack of reporting requirements, they posed a serious threat to healthcare entities. "These attacks typically involved the impersonation of senior executives, coercing staff members into initiating unauthorized wire transfers or disclosing sensitive data," he said. This trend underscores the adaptability of cybercriminals in exploiting various communication channels. "We are now getting anecdotal evidence of the use of AI and deepfake techniques to enhance these attacks," Moore said. The second developing trend involves the constant evolution of ransomware and its double and triple extortion flavors, according to Moore. "What's noteworthy is the growing recognition within the industry of the direct impact these attacks have on patient care and patient outcomes" he said. Hospitals, in particular, bore the brunt of these malicious campaigns. Some estimates put the number of hospitals affected by ransomware attacks this year to be around 300 hospitals, Moore said. "While there's a positive aspect in the increasing acknowledgment of this issue, the downside is that these attacks continue to jeopardize patient well-being." Chaudhuri said he anticipates an increase in targeted phishing attacks exploiting human error in 2024. "Additionally, we might see increased attacks exploiting internet of medical things devices as these become more integrated into healthcare services," he said. "Attackers are likely to exploit the interconnected nature of these devices, leading to more sophisticated breaches." Welcome to the Ways of Thinking competency quadrant. The competency definitions can be found below. Feel free to expand the section and read the definitions. Following the definitions, there is a section for resources with external links to the resources listed for you. Utilize this resource section as a preliminary starting point for the development of your leadership and professional development journey. Decision-Making & Problem-Solving Defines a problem or issue; identifies its potential causes; specifies a desired outcome; employs critical, practical, and creative thinking skills to generate possible solutions; and identifies and implements effective criteria for choosing amongst possible solutions. Ethics Understanding standards and expectations for personal and professional ethical behavior by acting in accord with an appropriate set of social norms, beliefs, and cultural values (e.g., trustworthiness, respect, responsibility, fairness, caring, and citizenship). Considers implications of actions and shows an awareness of the need to hold one’s self to a higher standard. Idea Generation Developing new and/or novel ideas through critical thinking and creative processes that address issues and/or lead to change. Reflection & Analytical Reasoning Considering the past and learning from successes and failures –own and others’ – to understand a situation, strategies used, and the impact of decisions. Employing critical, practical, and creative thinking skills within an ethical framework to connect disparate information; understanding the context of the situation from multiple perspectives; synthesizing information; being open-minded and flexible while considering multiple possible solutions. Systems Thinking & Planning Identifying tasks and setting deadlines to design, evaluate, and implement strategies to answer questions or achieve desired goals. Assessing a situation, organization, or network through examination of the linkages, interconnections, and/or interactions of it component parts – both internal and external – to better understand how it works, to be able to navigate through ripple effects of others’ decisions, and to make decisions that consider impact on a larger network or system. Resources
For specific resources, search the Experiences Database Technology’s exponential development and use in healthcare provides potentially significant benefits for behavioral health patients but also raises ethical and compliance concerns. The most latest technological advance involves the use of artificial intelligence (AI). Unfortunately, laws, rules, and regulations do not change as quickly as technology. Compliance professionals will want to keep in close contact with departments considering using AI—including behavioral health—for both ethical and confidentiality concerns. Mental health stigma is alive and well and can create issues for employment and other activities for those suffering from mental health conditions. When compliance collaborates with departments such as behavioral health, those concerns can be minimized. The pandemic brought mental health issues to the forefront. Both the Biden administration and the Substance Abuse and Mental Health Services Administration (SAMHSA) have put forth plans to address identified issues. The Biden administration is working to Boost insurance coverage for mental health, while SAMHSA is working to strengthen the release of information requirements, especially for substance use disorder. The use of AI will factor in both plans as a potentially cost-effective way to address mental health concerns. AI benefitsSo, what is AI? According to IBM, “Artificial intelligence leverages computers and machines to mimic the problem-solving and decision-mailing capabilities of the human mind.”[1] As more commonly understood, it is having computers “thinking” like humans. According to research, AI appears to provide several improvements in treating mental health conditions. A study published in the Journal of Medical Internet Research found that AI “was associated with significant improvements in substance use, confidence, cravings, depression, and anxiety.”[2] The authors believe that the benefits of AI are the ability to compare and analyze large amounts of data as well as increase “equity and access” to mental health treatment.[3] One disadvantage identified is predictability with ethnic groups who may not have access to mental healthcare. Lack of access would lead to a lack of data for AI to analyze, making it less likely to accurately predict issues in that population.[4] Several studies on groups where data is available found a high level of accuracy in predicting suicidal thoughts as well as significant mental health issues.[5] A Vanderbilt study found that with access to medical records information, demographics, and admissions information, AI had an 80% accuracy rate in predicting whether an individual would die by suicide.[6] With all these benefits, it seems that AI should be pursued; however, at the same time, there are numerous ethical and privacy issues to be considered and addressed. Ethical concernsAn excellent example of potential privacy and ethical issues is the Dinerstein v. Google case.[7] In this case, Matt Dinerstein sued because his de-identified information was given to Google under a data use agreement as part of a research project. Google has access to information from other applications that would allow his information to be re-identified. As electronic records and health apps gather information on individuals’ health information, and smartphones can geolocate individuals, the ability to re-identify individuals becomes more of a reality and de-identification more of a myth.[8] Another ethical situation involved a study identifying that an organization used AI to provide counseling without telling patients.[9] In this case, a mental health entity used a chatbot to provide treatment for 4,000 patients without informing them that a human was not providing that service.[10] There is also concern that AI may be vulnerable to misuse—intentional or not—by changes in how the data is input into the system.[11] As previously mentioned, a gap exists between technological advances and regulations attempting to catch up with those rapid changes. The U.S. Food and Drug Administration (FDA) stated that the current regulatory process is “not equipped to handle the speed of change” which is needed for ensuring safety and effectiveness.[12] Additionally, both the U.S. Department of Health and Human Services Office for Civil Rights (OCR) and SAMHSA have promised new regulations; however, several years later, none have been forthcoming. Compliance professionals can only hope that when new regulations are published, they will also address AI concerns. This lack of regulation leaves healthcare entities to figure out safety, ethical, and privacy issues on their own. It is crucial for organizations to address these issues. For AI to perform those functions, it must analyze data from multiple sources, including the patient’s medical record. This places a great deal more information about a patient in the electronic environment, which has proven vulnerable to attack by hackers. Ransomware is prevalent within healthcare due to the large amount of information available today. Credit card companies used to be the avenue of attack, but changes in regulation and practices have made it more difficult to access a person’s information. AI can increase not only the amount of information but also the number of patients’ information available, making it a likely further target of ransomware attacks. Several ethical and legal concerns have been identified from studies performed by various researchers, including those from the World Health Organization (WHO). Most of these studies were conducted following the Trump administration’s executive order covering five areas related to AI.[13] The idea behind the executive order was to increase the development and use of AI in healthcare. WHO identified several concerns with the use of AI and listed some ethical principles that need to be applied when developing and using AI. The concerns centered around data bias that could lead to inaccurate information being used by healthcare practitioners; information being provided to an end user could be erroneous but appear to be accurate and legitimate; and lack of consent for the use of patient information used in training an AI system.[14] The ethical principles include:
In other words, anyone using AI should address the concerns identified, find ways to minimize or eliminate bad data or data being used inappropriately and start gathering a broader spectrum of data so that all populations are included in the benefits of AI. Compliance and AI riskHow do compliance professionals help guide the ethical use of AI and identify ways to protect patient privacy? Initially, compliance should be involved in drafting policies to keep up with the rapidly changing technological environment in mental health. Compliance professionals can also provide education on the benefits and detriments of AI and how to avoid the pitfalls. The use or potential use of AI should be included in the annual compliance risk assessment and/or enterprise risk assessment. Monitoring and auditing should occur to ensure the ethical use of AI and the protection of patient information. From a privacy perspective, new de-identification methods may need to be created to minimize the ability to re-identify a patient using multiple sources of information. It will be important to monitor OCR regulations to see how they strengthen patient privacy protection and implement any new regulations as quickly as possible. Working with OCR and SAHMSA for realistic and beneficial methods and regulations should also be a priority. As compliance professionals, we are charged with ensuring ethical conduct of our healthcare institutions and protecting our patients’ information to the fullest extent possible. These steps will help us close the gap with the rapidly changing technological environment taking place. Takeaways
1 IBM, “What is artificial intelligence (AI)?” accessed October 25, 2023, https://www.ibm.com/topics/artificial-intelligence#:~:text=Artificial%20intelligence%20leverages%20computers%20and,capabilities%20of%20the%20human%20mind. 2 Jessica Kent, “What Role Could Artificial Intelligence Play in Mental Healthcare?” Health IT Analytics, April 23, 2021, https://healthitanalytics.com/features/what-role-could-artificial-intelligence-play-in-mental-healthcare. 3 Kent, “What Role Could Artificial Intelligence Play in Mental Healthcare?” Health IT Analytics. 4 Bernard Marr, “AI in Mental Health: Opportunities and Challenges In Developing Intelligent Digital Therapies,” Forbes, July 6, 2023, https://www.forbes.com/sites/bernardmarr/2023/07/06/ai-in-mental-health-opportunities-and-challenges-in-developing-intelligent-digital-therapies/?sh=7b1fa3055e10. 5 Marr, “AI in Mental Health: Opportunities and Challenges In Developing Intelligent Digital Therapies.” 6 Marr, “AI in Mental Health: Opportunities and Challenges In Developing Intelligent Digital Therapies.” 7 Dinerstein v. Google, LLC, No. 20-3134 (7th Cir. Jul. 11, 2023). 8 Sara Gerke, Timo Minssen, and Glenn Cohen, “Chapter 12 – Ethical and legal challenges of artificial intelligence-driven healthcare,” in Artificial Intelligence in Healthcare (London: Academic Press, 2020): 295–336, https://doi.org/10.1016/B978-0-12-818438-7.00012-5. 9 Sabrina Moreno, “Growth of AI in mental health raises fears of its ability to run wild,” Axios, March 9, 2023, https://www.axios.com/2023/03/09/ai-mental-health-fears. 10 Moreno, “Growth of AI in mental health raises fears of its ability to run wild.” 11 Gerke, Minssen, and Cohen, “Ethical and legal challenges of artificial intelligence-driven healthcare.” 12 Moreno, “Growth of AI in mental health raises fears of its ability to run wild.” 13 Gerke, Minssen, and Cohen, “Ethical and legal challenges of artificial intelligence-driven healthcare.” 14 “Artificial intelligence in mental health research: new WHO study on applications and challenges,” World Health Organization, February 6, 2023, https://www.who.int/europe/news/item/06-02-2023-artificial-intelligence-in-mental-health-research--new-who-study-on-applications-and-challenges. 15 “Artificial intelligence in mental health research: new WHO study on applications and challenges.” *Barbara Vimont is Director of Corporate Compliance at Parkview Health in Fort Wayne, IN. The room hummed with giggles and mutters of “one, two, three, four, I declare a thumb war,” as a few dozen educators thumb-wrestled their way through a presentation on systems thinking. Sound strange? Imagine, then, doing it with your students to illustrate how preconceived notions can influence actions. Instructor Joan Yates, project manager for systems thinking in the Catalina Foothills School District in Tucson, Arizona, asked the teachers to thumb-wrestle for one minute with the goal of getting the most pins as possible. Most took that to mean the goal was to win. After all, a game requires a winner – right? In systems thinking, the answer is: Not necessarily. Yates pointed out that her instructions were to get the most pins; to actually get the most, the participants should have cooperated and taken turns getting pins, without a winner. The exercise is just one example of how teachers can introduce systems thinking concepts to students. It’s an approach that incorporates instructional tools to enhance learning about literature, history, current events, and science, and uses exercises to train students to think differently. In a nutshell, Yates says, systems thinking considers the relationship between the parts of a system, and the “dynamics those relationships produce.” A system can be anything – a novel, a historical event, a culture, a scientific formula. All are made up of different pieces that form the “system.” In systems thinking, you look at the whole of something, the individual parts of that whole, how those parts make the “whole” what it is, and how one action to a piece of the system can affect the entire thing. Change those habits Systems thinking in education helps develop students who can understand the value of other opinions, and see things from a different perspective, Yates says. Introducing mental modeling, which is ingrained assumptions that ultimately influence how we see things and what we do, can be a good place to start. The thumb-wrestling exercise, and others, such as asking students to fold their hands or cross their arms in the opposite way they normally do or walk up stairs starting with the opposite leg, encourages students to throw off their own mental models. They have to step out of their comfort zones and try new ways of looking at things. Yates says it’s important to take time to do these physical activities because the more senses that are engaged, the more likely someone will retain the material and really get out of those comfort zones. “It shocks people,” Yates says. “It discombobulates people enough that they physically feel. It gets them on more than one level. You increase the likelihood that someone will retain it, the more senses you engage.” Systems thinkers also develop certain “habits,” or ways of approaching problems and situations. The Waters Foundation, which supports systems thinking in schools, has 13 habits. If you use some of the systems thinking lessons and tools, students will start to develop these habits, but you can introduce them specifically. The habits of systems thinkers include: considering long and short-term consequences of actions (such as, if you have money, thinking both about what happens if you spend it immediately and if you put it in the bank); recognizing there might be unintended consequences to your actions; identifying the circular nature of complex cause and effect relationships (the bee buzzing around the flower is a system, where the bee needs the flower and the flower needs the bee); and looking at things from different angles and perspectives. Tools for teaching Practicing systems thinking in schools can be a big or small thing. In Yates’ Arizona district, systems thinking is integrated into all the classrooms, beginning in kindergarten. But there are a number of tools individual teachers can use to supply their students the benefits of a systems thinking approach. Useful for literature and social studies classes in particular is the “ladder of inference.” It helps students understand how they and others get to certain conclusions or form certain opinions (their own mental models). Literally a ladder, it starts at the bottom rung with what you know about yourself and works up: first you notice certain things, then you add your own meanings to what’s around you, then you develop beliefs based on those meanings, and finally, you doing something because of your beliefs. It’s a reinforcing loop, since the beliefs you develop are based on your personal experiences, and your beliefs affect what you notice about things and the meanings you apply. Use that ladder to analyze why a character does something. Take any character – say, Huck Finn – and start with what you know about him, what he does and notices in the story and how his experiences and background affect what he does. It’s a great way for students to understand how cultural and other experiences shape a character and why they might behave peculiarly, Yates says. Social studies teachers also can use this to study a character in history. A behavior over time graph is another systems tool to increase understanding. Simple line graphs that can be used with kindergartners on up, they look at what is changing and how it is changing. In English class, students do this in response to memorizing by examining how a character or situation changed over the course of several chapters. In social studies, a current events teacher can use it to supply students an understanding of how a world event unfolds. Take a newspaper article on a global issue and ask students to create a graph to illustrate the events in the story and what happened. Creating a deeper understanding Systems thinking isn’t just about the tools to help students see the world with a better lens; it also can supply them a greater grasp of why things happen a certain way. Things are circular in systems thinking, and recognizing the complex nature of cause-and-effect relationships can help students understand why things happen. One practice useful for students is called “fixes that fail.” Fixes that fail loops start with a problem and a solution to that problem. But rather than solving the problem, the solution creates an intended consequence, which reinforces the problem, perhaps making it even greater. Students can use this to examine the Vietnam war, for instance, showing how the United States’ actions led to greater problems rather than solving the original one. Yates says many current and historical events can fit into fixes-that-fail loops. Teachers also can have students look at global events in which bad fixes were avoided. Using systems thinking approaches in the classroom creates students who can see from another perspective and look deeper to why world events play out in certain ways. “If students develop those habits of thinking systemically, and they look at any global issue, they are going to ask different questions,” Yates says. “They are going to ask questions with a broader perspective.” When students leave her Arizona school district, Yates says, she hopes they take this way of thinking into everything they do. Author: Alexandra Moses
Do you use systems thinking in your classroom, and what benefits to students have you seen? How easy or difficult is it for them to throw off those mental models? Prashant Bansal is a leading technology sales professional and the business development director at L&T Technology Services. Minute observations can teach you the best lessons in life. When it comes to business development strategy, we can learn from examples around us. Some of the key insights I’ve picked up have surprisingly come from kickboxing, my newly discovered interest. The most important lesson I’ve learned is that you must pivot and change your strategy depending on your surroundings. When you’re boxing with a bag in the gym, you’re dealing with a familiar, stable environment. You can choose to practice certain moves or exercise different muscles without having to consider many external factors. But the moment you enter the ring with an opponent, you are stepping into the unknown. You don’t know which moves will land and which will miss until you test the waters. You need to be agile enough to take in new information and change your approach to meet the needs of a new environment. The same is true for entering a new market. Localization is key to developing a successful sales and marketing strategy. A tried-and-true formula in a developed nation might flop in an emerging market. It’s crucial to adapt your strategies to resonate with each local audience. Here are four other lessons I’ve learned from kickboxing that are also valuable in optimizing your sales strategy in a new market. 1. Find The Right AngleIn kickboxing, getting stuck in a rut—going for the same punches or kicks from the same angles, time after time—can lose you a fight. You always want to look for new moves and perspectives to overcome obstacles in changing circumstances. Similarly, in business, if a product has already had success in the U.S. or another developed market, don’t assume you can replicate the same roadmap in a developing economy. Evaluate your product roadmap, the plan that communicates a new product’s vision and strategic direction to key stakeholders, with location and context in mind. Develop your team’s knowledge of each regional market, and hire local domain experts who can advise you on how to tailor your product roadmap and your sales, marketing and delivery strategies to that specific market. For example, one company I’ve worked for initially partnered with an Indonesian company to sell its products and solutions in the APAC market. The Indonesian company brought in-depth knowledge of the region and tailor-made strategies, such as differentiated pricing models, that facilitated faster time to market. 2. Keep MovingComplacency is dangerous in kickboxing. If you stay still, lose your focus or fail to adapt, you may get the wind knocked out of you. You must keep moving constantly and adapting your strategy as you go. In the U.S. and other developed nations, for example, consumers tend to be well-versed in technology. They have grown up using tech in their everyday lives and are generally comfortable adopting new products. If we are marketing a product that uses AR, VR or 5G technology, such as a home security solution with 5G capabilities, there isn’t a huge learning curve for U.S. consumers. But if we want to sell the same product in countries that aren’t as familiar with new tech, we have to incorporate a lot more education into our digital marketing strategies. Instead of just pushing a product without context, we need to start with the basics in our marketing: how the technology works, what problem it solves and why this particular product is valuable. 3. Follow The RulesIt doesn’t matter if you’re the best fighter in the world; if you break too many rules, you’ll lose the match. It’s crucial to learn, study and revisit the rules of the game long before the first round begins. You don’t want to supply up a win simply because you didn’t understand or respect the regulations. In the same way, you could release an incredible product that fails because you neglected to do your due diligence on legal and compliance issues. Before launching a product in a new market, have an expert review its legal and compliance documentation and determine the changes you need to make. Companies headquartered in the U.S. often use their local legal teams as a default in case of any dispute, but this can be problematic if you are expanding into emerging economies. For instance, our products often come with strict service-level agreements and key performance indicators, stating that a device or software will deliver a particular solution or response time. But we have to be careful when drawing up contracts in different parts of the world. We can’t recycle the language we use in the U.S. because the network time in a developing country won’t be the same as it is in North America. It’s important to be aware of these limitations and localize our terms and conditions with specificity. 4. Study Your CompetitorIt is important to focus on your strengths; however, it is equally important to monitor your competitor’s weaknesses. In the words of boxing legend Manny Pacquiao, “If you work hard in training, the fight is easy.” Bring the discipline of training to your business. Concentrate on developing market-specific product roadmaps, creative marketing strategies and indisputable legal frameworks, but also pay attention to players already in the market. What can you learn from their actions, successes and missteps? What opportunities can you uncover? A competitor may have been in business for a long time, but something may be holding them back from being the market leader. Identify that shortcoming and what it means for your business. When you introduce a product to a new market, you have to understand your surroundings and how they affect your strategy. You can’t just copy and paste the sales and marketing plans that worked on the other side of the world. Just like in kickboxing, you must learn to pivot and adapt to the needs of each new environment. Forbes Business Development Council is an invitation-only community for sales and biz dev executives. Do I qualify? 
Scott Groves
Licensed Professional Counselor, Scott Groves, MS, LPC "I'm just going through the motions". "I feel hopeless". "What's the purpose"? These thoughts and feelings are all too common. Relationships suffer, self-doubt creeps in and you begin to worry about things that were never an issue years ago. The idea that life should somehow be different than it is now affects most people at some point. How do we deal with the stress this causes us and the people we love? Reaching out to someone for help can be difficult for anyone. Women tend to be more open to the idea of help and men are typically much more reluctant but we all want to know that the person we reach out to actually understands us. "I'm just going through the motions". "I feel hopeless". "What's the purpose"? These thoughts and feelings are all too common. Relationships suffer, self-doubt creeps in and you begin to worry about things that were never an issue years ago. The idea that life should somehow be different than it is now affects most people at some point. How do we deal with the stress this causes us and the people we love? Reaching out to someone for help can be difficult for anyone. Women tend to be more open to the idea of help and men are typically much more reluctant but we all want to know that the person we reach out to actually understands us. 
Amy Adair
Licensed Professional Counselor, MS, RN, LPC Verified As a counselor and a nurse, I have spent my whole career helping people struggling through life's difficult journeys. Are you struggling with the lingering effects of abuse, neglect, trauma, divorce, loss or unwanted change? Though we can't change the circumstances, we can work together to Boost how you express yourself, make decisions, resolve conflicts, manage stress and eliminate thoughts and behaviors that keep you from living your best life. As a counselor and a nurse, I have spent my whole career helping people struggling through life's difficult journeys. Are you struggling with the lingering effects of abuse, neglect, trauma, divorce, loss or unwanted change? Though we can't change the circumstances, we can work together to Boost how you express yourself, make decisions, resolve conflicts, manage stress and eliminate thoughts and behaviors that keep you from living your best life. 
Amber M Prather
Licensed Professional Counselor, MAMFT, LPC I enjoy working with clients wherever they may find themselves on life's journey and offer a safe and accepting therapeutic relationship to explore their concerns. My passion is to assist clients on their journey toward wellness, healing and growth. My therapeutic approach is client focused, collaborative, and holistic. I enjoy working with clients wherever they may find themselves on life's journey and offer a safe and accepting therapeutic relationship to explore their concerns. My passion is to assist clients on their journey toward wellness, healing and growth. My therapeutic approach is client focused, collaborative, and holistic. 
Vonna Lovett
Marriage & Family Therapist, LMFT Verified It is my desire to join with you in seeking release of past struggles and looking to the future. With families, couples, and individuals I can offer resources to assist in the desires for healing and wholeness while reaching the full potential in your life. If that journey deals with grief/loss, addictions, trauma, divorce, family challenges, or individual challenges, I would consider meeting you where you are on that journey a privilege. It is my desire to join with you in seeking release of past struggles and looking to the future. With families, couples, and individuals I can offer resources to assist in the desires for healing and wholeness while reaching the full potential in your life. If that journey deals with grief/loss, addictions, trauma, divorce, family challenges, or individual challenges, I would consider meeting you where you are on that journey a privilege. 
Michele M Burris
Licensed Professional Counselor, MEd, LPCS Ms Michele Burris, MED, MED, LPC, LPCS - 12 Years experience in counseling and 21 years in Life Coaching. She offers a hybrid in-person/telehealth counseling experience. She is a Veteran PTSD Recovery Specialist - Veteran Services Specialist, as well as, skilled psychotherapist. Ms Michele Burris obtained a MEd in Adult Education and a second MEd in Guidance and Counseling from the UC of Oklahoma, 1991 and 2012 in Edmond, Oklahoma; Oklahoma Licensed Professional Counselor 5619 since 12/2014. Army National Guard Behavioral Health Specialist Trained - 5yrs, Army Master Resilience Training Trainer - University of Pennsylvania Program. Ms Michele Burris, MED, MED, LPC, LPCS - 12 Years experience in counseling and 21 years in Life Coaching. She offers a hybrid in-person/telehealth counseling experience. She is a Veteran PTSD Recovery Specialist - Veteran Services Specialist, as well as, skilled psychotherapist. Ms Michele Burris obtained a MEd in Adult Education and a second MEd in Guidance and Counseling from the UC of Oklahoma, 1991 and 2012 in Edmond, Oklahoma; Oklahoma Licensed Professional Counselor 5619 since 12/2014. Army National Guard Behavioral Health Specialist Trained - 5yrs, Army Master Resilience Training Trainer - University of Pennsylvania Program. 
Gaybra L Schultz
Clinical Social Work/Therapist, MSW, LCSW, PLLC Verified We are made for connection with others and have the desire to feel seen and heard. You may be going through a life change and are struggling with fear, grief, shame, or worry. You may be exhausted living in a constant high-stress mode. You may be feeling overwhelmed with your circumstances or simply just not feel like yourself. You want to feel that hope and change is possible. It is. The human spirit is truly remarkable. We can't stop life from bringing us expected or unexpected life situations but we can learn new strategies to approach and respond to these moments through discovering more about ourselves and learning new skills. We are made for connection with others and have the desire to feel seen and heard. You may be going through a life change and are struggling with fear, grief, shame, or worry. You may be exhausted living in a constant high-stress mode. You may be feeling overwhelmed with your circumstances or simply just not feel like yourself. You want to feel that hope and change is possible. It is. The human spirit is truly remarkable. We can't stop life from bringing us expected or unexpected life situations but we can learn new strategies to approach and respond to these moments through discovering more about ourselves and learning new skills. 
Melody R Daniels
Licensed Professional Counselor, JD, MA, LPC Verified As a counselor, it is my passion to assist those through the struggles along life's journey. We are all living through difficult times which can make us feel isolated, alone, anxious depressed or just extra. In addition, these stressors can also increase how we experience and perceive the stressors of everyday life as well as how we communicate and interact in relationships with those we love the most. If you want to gain some control over your emotions so that you are living the life you deserve, then therapy could be the answer for you and I would be honored to be on this journey with you. As a counselor, it is my passion to assist those through the struggles along life's journey. We are all living through difficult times which can make us feel isolated, alone, anxious depressed or just extra. In addition, these stressors can also increase how we experience and perceive the stressors of everyday life as well as how we communicate and interact in relationships with those we love the most. If you want to gain some control over your emotions so that you are living the life you deserve, then therapy could be the answer for you and I would be honored to be on this journey with you. 
Lawrence M Ackerman
Licensed Professional Counselor, MA, LPC Thank you for taking the first step in seeking out counseling. Many clients are having trouble navigating through a whole bunch of life and personal issues. It may include difficulties with relationships, problems on the job, unresolved issues from the past, grief and loss, mood disorders, impulse control, uncertainties about the future. Some people are reluctant to seek out a professional thinking how can they understand my issues? What do I talk about? Can I really open up to them about some of my deepest issues? Well, I want you to know, that whatever it is, you have come to the right place. I look forward to guiding you. Thank you for taking the first step in seeking out counseling. Many clients are having trouble navigating through a whole bunch of life and personal issues. It may include difficulties with relationships, problems on the job, unresolved issues from the past, grief and loss, mood disorders, impulse control, uncertainties about the future. Some people are reluctant to seek out a professional thinking how can they understand my issues? What do I talk about? Can I really open up to them about some of my deepest issues? Well, I want you to know, that whatever it is, you have come to the right place. I look forward to guiding you. 
Rebecca Thompson
Licensed Professional Counselor, MSCP, LPC Not accepting new clients
At some point, we often find ourselves struggling to get past a problem, whether that problem is with an important relationship, or an inner emotional struggle. I enjoy working with others to understand how one came to be in such conflict and how one can move from old ways of coping to newer healthier ways. I believe working from a relational perspective is an important way to create an atmosphere of trust. I strive to help my patients better understand their thoughts, feelings, and actions. The more we know about ourselves the more freedom we have to make better choices in work, play, and relationships. At some point, we often find ourselves struggling to get past a problem, whether that problem is with an important relationship, or an inner emotional struggle. I enjoy working with others to understand how one came to be in such conflict and how one can move from old ways of coping to newer healthier ways. I believe working from a relational perspective is an important way to create an atmosphere of trust. I strive to help my patients better understand their thoughts, feelings, and actions. The more we know about ourselves the more freedom we have to make better choices in work, play, and relationships. 
Christa Carrillo Brown
Licensed Professional Counselor, MEd, LPC, PLLC Hello, my name is Christa Brown and I have the privilege of being in a field helping others. I will paraphrase an old Irish proverb to supply an example of how I see the counseling relationship. "A tree alone in a storm may not have the best chance to survive or thrive, although, a tree that is amongst the forest in the storm has the best chance of survival and thriving." My hope is that in the counseling relationship my client's learn to draw from their own strength and the support of others to survive and thrive when they encounter storms in their lives. Hello, my name is Christa Brown and I have the privilege of being in a field helping others. I will paraphrase an old Irish proverb to supply an example of how I see the counseling relationship. "A tree alone in a storm may not have the best chance to survive or thrive, although, a tree that is amongst the forest in the storm has the best chance of survival and thriving." My hope is that in the counseling relationship my client's learn to draw from their own strength and the support of others to survive and thrive when they encounter storms in their lives. 
Lynda Pauwels Osborn
Clinical Social Work/Therapist, LCSW, MSW Verified The ideal clients have made an honest self-assessment of their lives, acknowledged an awareness of issues or concerns that are robbing them of their joy, and are committed to developing and integrating new strategies for meeting life's challenges with renewed hope and optimism. The ideal clients have made an honest self-assessment of their lives, acknowledged an awareness of issues or concerns that are robbing them of their joy, and are committed to developing and integrating new strategies for meeting life's challenges with renewed hope and optimism. 
Karen Mosshammer
Licensed Professional Counselor, MS, LPC, MBA, CCTS Verified Not accepting new clients
I not currently accepting new patients. Seeking individuals ready to explore themselves, to challenge their negative thoughts, to evaluate their emotions, to believe in their core value and are willing to allow me to guide them to what they believe is a more fulfilling life. I not currently accepting new patients. Seeking individuals ready to explore themselves, to challenge their negative thoughts, to evaluate their emotions, to believe in their core value and are willing to allow me to guide them to what they believe is a more fulfilling life. 
Dr. Kimberly Burdine
Psychologist, PhD Verified My work is centered in imagining liberation and healing free from anti-Black racism, colonialism, state violence, cissexism, ableism, capitalism, xenophobia and any form of systemic oppression. I am committed to the healing and liberation of Black folx, particularly those in the margins. Queer, disabled, immigrant, women, young, Black folx have a right to be well and access quality mental health care. As a queer Black woman, who has benefitted from community, healing, and affirmation, it is meaningful and spiritually fulfilling to offer support to folx with similar identities. My work is centered in imagining liberation and healing free from anti-Black racism, colonialism, state violence, cissexism, ableism, capitalism, xenophobia and any form of systemic oppression. I am committed to the healing and liberation of Black folx, particularly those in the margins. Queer, disabled, immigrant, women, young, Black folx have a right to be well and access quality mental health care. As a queer Black woman, who has benefitted from community, healing, and affirmation, it is meaningful and spiritually fulfilling to offer support to folx with similar identities. 
Mary Lou Tabers, LMFT, PLLC
Marriage & Family Therapist, MA, LMFT The client I see is one that realizes there is a healthier way to have relationship with others, and I can show you how to find the person that you are, so others do not take away. You may be the client that feels stuck, and I can help you to process this transitional time, and will help you to find coping skills needed so you can direct your energies toward new goals. If you are a client grieving the loss of a loved one, a vocation, a dream, and you realize the need to grieve, the time is now to find healing. The client I see is one that realizes there is a healthier way to have relationship with others, and I can show you how to find the person that you are, so others do not take away. You may be the client that feels stuck, and I can help you to process this transitional time, and will help you to find coping skills needed so you can direct your energies toward new goals. If you are a client grieving the loss of a loved one, a vocation, a dream, and you realize the need to grieve, the time is now to find healing. 
Mark D Heaney
Licensed Professional Counselor, D, Min, LPC Verified I work with individuals,couples and families who desire to solve emotional, relational, physical and spiritual challenges. I will guide you to reach life goals, solve relational problems, overcome emotional issues, and achieve Inner Peace. You will discover your strengths and sharpen your abilities as you create a positive vision for the future. I am very excited to work with you. Call and ask for a free consultation. I work with individuals,couples and families who desire to solve emotional, relational, physical and spiritual challenges. I will guide you to reach life goals, solve relational problems, overcome emotional issues, and achieve Inner Peace. You will discover your strengths and sharpen your abilities as you create a positive vision for the future. I am very excited to work with you. Call and ask for a free consultation. 
Dr. Wendy Fine-Thomas
As a clinical psychologist for more than twenty-five years, I have become skilled at helping successful, professional women move forward in life. I work with women who “have it all” but feel like imposters in their own lives. I foster a calm yet intellectually challenging environment for women - physicians, therapists, and executives - to learn about themselves. We explore how professional roles impact mothering, friendships, and intimacy. I utilize my skills, education, and professional resources to help women move beyond feeling emotionally paralyzed to create a more peaceful life of meaning and connection. As a clinical psychologist for more than twenty-five years, I have become skilled at helping successful, professional women move forward in life. I work with women who “have it all” but feel like imposters in their own lives. I foster a calm yet intellectually challenging environment for women - physicians, therapists, and executives - to learn about themselves. We explore how professional roles impact mothering, friendships, and intimacy. I utilize my skills, education, and professional resources to help women move beyond feeling emotionally paralyzed to create a more peaceful life of meaning and connection. 
Calvin Smith, LPC
Licensed Professional Counselor, MS, LPC Verified Online Therapy focus due to Covid-19 precautions. Be confident you’re safe while you get professional care.I specialize in helping people work through their pain, worry, confusion, and anxiety. Sometimes it isn't easy to know where to start or how to ask for help. It is equally challenging to know where to go to get it. You can start here. Schedule a private and confidential appointment in a place were you can work on resolving the challenges you are experiencing. Whether individuals, couples, children/teenagers, employees, contractors, business men, clergy, great or distressed background, you are welcome here as a place to start. Online Therapy focus due to Covid-19 precautions. Be confident you’re safe while you get professional care.I specialize in helping people work through their pain, worry, confusion, and anxiety. Sometimes it isn't easy to know where to start or how to ask for help. It is equally challenging to know where to go to get it. You can start here. Schedule a private and confidential appointment in a place were you can work on resolving the challenges you are experiencing. Whether individuals, couples, children/teenagers, employees, contractors, business men, clergy, great or distressed background, you are welcome here as a place to start. 
Kelcy Eckels
Licensed Professional Counselor, MA, LPC Hey, people-pleaser, do you feel like you're drowning in other people's needs? Are you constantly worrying about not causing problems & bending over backward to fix everyone's problems while putting your needs on the back burner? Are you too harsh on yourself? Do you question yourself to extreme confusion about what you know & want? Maybe your heart wants to recover your sense of self from past one-sided relationships. You may even have big emotions about events that don't always warrant such big feelings. If this is you, keep memorizing to see how we can work together. Hey, people-pleaser, do you feel like you're drowning in other people's needs? Are you constantly worrying about not causing problems & bending over backward to fix everyone's problems while putting your needs on the back burner? Are you too harsh on yourself? Do you question yourself to extreme confusion about what you know & want? Maybe your heart wants to recover your sense of self from past one-sided relationships. You may even have big emotions about events that don't always warrant such big feelings. If this is you, keep memorizing to see how we can work together. 
Rita Ngozi Offiah
Licensed Professional Counselor, MHR, LPC Are you a mother or father who is struggling with finding balance in your everyday life especially parenthood and or career? Do you feel like you're not meeting expectations you've set for yourself or allowed others to set for you? Are you struggling with communicating with your spouse or significant other? Are you a teenager or young adult who feels misunderstood and can't seem to relate to the adults in your life? Do you feel like your opinions are not valued? Are you Getting ready to transition to adulthood but feels unprepared to handle adult issues and problems? Are there transitions causing you emotional distress? Are you a mother or father who is struggling with finding balance in your everyday life especially parenthood and or career? Do you feel like you're not meeting expectations you've set for yourself or allowed others to set for you? Are you struggling with communicating with your spouse or significant other? Are you a teenager or young adult who feels misunderstood and can't seem to relate to the adults in your life? Do you feel like your opinions are not valued? Are you Getting ready to transition to adulthood but feels unprepared to handle adult issues and problems? Are there transitions causing you emotional distress? See more therapy options for 73142 Nearby Searches for 73142How can I find a therapist in 73142?Search for nearby therapists or counselors by inputting your city, town, or suburb; or zip code; or a provider’s name into the search bar. From there, you can filter providers by the issues they treat, cost, insurance, gender, and other factors to find providers who are well-suited to your needs. To navigate between locations within the same country, enter a new city or zip code into the search bar.
Learn more about how to find a therapist. Is online therapy a good option?Therapy conducted online can be just as effective as in-person therapy, as long as there is a strong alliance between the client and the therapist. To find a therapist who provides telehealth services to clients in your area, click “Online Therapy” on the directory homepage and search by your city or town or your zip code.
What’s the difference between a psychologist, a therapist, and a counselor?Therapists, psychologists, and counselors are all licensed mental health professionals. In the US, psychologists have earned a doctoral degree. The terms “therapist” and “counselor” are used somewhat interchangeably, but generally therapists offer longer-term, mental health care, while counselors offer shorter-term care that may focus on one domain, such as marriage, career, or academic challenges.
What type of therapist is right for me?Clients should consider factors such as insurance coverage and their primary reason(s) for seeking therapy to determine the type of professional best suited to their needs. Someone struggling with mental health challenges such as depression or anxiety, for example, may wish to seek out a clinical psychologist or therapist, while someone navigating career obstacles or marital upheaval may benefit from seeing a counselor who can offer short-term, targeted support. Is everyone in the Psychology Today Therapy Directory a licensed therapist?The Psychology Today directory lists providers who offer legitimate mental health services to the public, including psychologists, psychiatrists, social workers, and counselors. Many have been licensed by the country or state where they practice; providers whose license or primary credential has been verified by Psychology Today are signified by a “Verified” symbol. Some clinicians or organizations provide services for which their state or country does not offer licenses, such as pastoral counseling. They may be selectively included without the “Verified” seal.
What type of therapy is right for me?The type of therapy best suited to a particular individual depends on several factors, including their primary reason for seeking therapy, their preferred timeline (some therapy types last for a set number of sessions, while others are open-ended), and their personality and preferences—some may prefer a more structured approach. For many individuals, multiple types of therapy could provide a good fit.
Is online therapy cheaper than in-person therapy?Many therapists charge the same amount for online therapy as they do for in-person therapy—though clients may still find this cost-effective if it cuts down on their transportation costs. Health insurance plans often offer equivalent coverage for online and in-person therapy; indeed, in many places, they are legally required to do so. Text-based or on-demand therapy apps may be cheaper than traditional one-on-one psychotherapy; however, the practice may be less effective and is not likely to be covered by insurance. 
Allison Carey
Marriage & Family Therapist, LMFT, LFYP- Keene, NH 03431 (Online Only)
Only accepting for individual therapy at this time. I work with people who are seeking a systemic, strengths-based, collaborative approach. My clients are primarily adolescents and adults experiencing relationship difficulties, parenting issues, marital distress, academic and behavioral difficulties, trauma symptoms and/or depressive & anxious moods. Those seeking to reduce daily stress and overwhelm, increase relationship satisfaction, and discover family patterns are a good fit for my approach. Only accepting for individual therapy at this time. I work with people who are seeking a systemic, strengths-based, collaborative approach. My clients are primarily adolescents and adults experiencing relationship difficulties, parenting issues, marital distress, academic and behavioral difficulties, trauma symptoms and/or depressive & anxious moods. Those seeking to reduce daily stress and overwhelm, increase relationship satisfaction, and discover family patterns are a good fit for my approach. 
Skyler Dietzman
Clinical Social Work/Therapist, LCSW Keene, NH 03431 (Online Only)
I am passionate about working with individuals with chronic stress, anxiety, self-doubt, burn out, and relationship difficulties. My clients are often experiencing major life shifts/changes. They are people pleasers, caregivers, helpers and highly sensitive individuals who want to Boost how they feel but have trouble prioritizing themselves. They want to uncover the hidden version of their best self and are ready to build confidence, practice self-compassion, Boost their relationships and put their needs first. I am passionate about working with individuals with chronic stress, anxiety, self-doubt, burn out, and relationship difficulties. My clients are often experiencing major life shifts/changes. They are people pleasers, caregivers, helpers and highly sensitive individuals who want to Boost how they feel but have trouble prioritizing themselves. They want to uncover the hidden version of their best self and are ready to build confidence, practice self-compassion, Boost their relationships and put their needs first. 
Christine Chamberlin
Psychologist, PhD Verified I work with individual adults seeking to clarify their life path, and who are up for the challenge of taking the necessary steps towards change. You may be encountering life transitions, stress, anxiety, or relationship difficulties, which may extend to a desire for a more comfortable or inspiring living or work environment. My primary interest is to empower you to clarify what you want, build confidence and self-nurturing skills, and manifest potential; in both you and your environment. I utilize techniques aimed at bringing forth what it is you truly desire and need, to live authentically. I work with individual adults seeking to clarify their life path, and who are up for the challenge of taking the necessary steps towards change. You may be encountering life transitions, stress, anxiety, or relationship difficulties, which may extend to a desire for a more comfortable or inspiring living or work environment. My primary interest is to empower you to clarify what you want, build confidence and self-nurturing skills, and manifest potential; in both you and your environment. I utilize techniques aimed at bringing forth what it is you truly desire and need, to live authentically. 
Carey Bluhm
Psychologist, PhD Verified I have training and experience in working successfully with a wide range of issues and ages, with individuals, families, couples and children. How might you choose the best counselor for you? Do you already know whether age and gender are important factors? Do you most of all need a specific time slot? Do you need someone who takes your insurance (or you can afford if you have none)? Does your prospective counselor do a specific kind of therapy you heard was good? You probably are concerned that the counselor will understand you and your specific problem or issues, but that is something you'll only be able to learn when you meet them. Has this counselor worked successfully with people with my problem or my circumstances? I have training and experience in working successfully with a wide range of issues and ages, with individuals, families, couples and children. How might you choose the best counselor for you? Do you already know whether age and gender are important factors? Do you most of all need a specific time slot? Do you need someone who takes your insurance (or you can afford if you have none)? Does your prospective counselor do a specific kind of therapy you heard was good? You probably are concerned that the counselor will understand you and your specific problem or issues, but that is something you'll only be able to learn when you meet them. Has this counselor worked successfully with people with my problem or my circumstances? 
Tricia McLeod
Not accepting new clients
I compassionately assist people struggling with a wide variety of issues and stressors, while specializing in anxiety, gender identity and sexuality, trauma, substance use, and relationship issues. I am a down to earth, intuitive therapist and provide counseling in a safe, practical and non-judgmental manner. I utilize a variety of therapeutic approaches tailored to each person's specific needs and goals. I truly believe in a person's ability to heal and recover and enjoy working collaboratively to meet people where they are at in their own process of change. I compassionately assist people struggling with a wide variety of issues and stressors, while specializing in anxiety, gender identity and sexuality, trauma, substance use, and relationship issues. I am a down to earth, intuitive therapist and provide counseling in a safe, practical and non-judgmental manner. I utilize a variety of therapeutic approaches tailored to each person's specific needs and goals. I truly believe in a person's ability to heal and recover and enjoy working collaboratively to meet people where they are at in their own process of change. 
Stephanie B Kimber
Counselor, MA, LCMHC Verified Keene, NH 03431 (Online Only)
Are you facing a stressful life transition, feeling a lack of purpose, or having difficulty making yourself a priority in your life? Perhaps you are processing trauma you have experienced? Struggling with a marital or relationship issue…or wondering about whether or how to navigate the delicate process of ending a long-term relationship and moving forward? If so, I invite you to consider taking the time to pause and reflect on what you need from therapy as you contemplate the path ahead. Are you facing a stressful life transition, feeling a lack of purpose, or having difficulty making yourself a priority in your life? Perhaps you are processing trauma you have experienced? Struggling with a marital or relationship issue…or wondering about whether or how to navigate the delicate process of ending a long-term relationship and moving forward? If so, I invite you to consider taking the time to pause and reflect on what you need from therapy as you contemplate the path ahead. 
Dr. Brian Quigley-Peak Experiences Counseling
Additionally, I have specialized expertise working with Depression & Anxiety; Athletes; Law Enforcement Professionals; Young Adults; Actors, Dancers, & Other Performers; Couples / Relationship Difficulties; Grief & Loss; Managing Stress & Life Transitions. Expertise, deeply caring, experienced, collaborative & open, down to earth- these describe me as a licensed psychologist. I'm an especially good fit if you're looking to avoid the mystery, confusion, and awkwardness sometimes associated with therapy. If you’re struggling with difficult emotions or challenging life circumstances, like many of us you may be feeling some unease about getting help. Well, I'm so glad you're here right now taking the courageous step of prioritizing your well-being! I'm confident that from my approach, therapy together will be wonderfully transformational, restorative, and life changing. You got this! Additionally, I have specialized expertise working with Depression & Anxiety; Athletes; Law Enforcement Professionals; Young Adults; Actors, Dancers, & Other Performers; Couples / Relationship Difficulties; Grief & Loss; Managing Stress & Life Transitions. Expertise, deeply caring, experienced, collaborative & open, down to earth- these describe me as a licensed psychologist. I'm an especially good fit if you're looking to avoid the mystery, confusion, and awkwardness sometimes associated with therapy. If you’re struggling with difficult emotions or challenging life circumstances, like many of us you may be feeling some unease about getting help. Well, I'm so glad you're here right now taking the courageous step of prioritizing your well-being! I'm confident that from my approach, therapy together will be wonderfully transformational, restorative, and life changing. You got this! 
Allen Penrod
Drug & Alcohol Counselor, MA, MS, MLADC Verified I'm a professional thinking partner , a contractor of sorts. Offices in Keene, NH. Treatment for all types of mood related difficulties, especially those related to stress or trauma, PTSD, anxiety, depression, and substance abuse. Insurance is not a factor. You and I decide what thinking together is worth to you. Thinking together can include on-line and phone consultations. I don't know what you need to enjoy life more, but I'm willing to bet you do. We will figure that out, and make it happen. I'm a professional thinking partner , a contractor of sorts. Offices in Keene, NH. Treatment for all types of mood related difficulties, especially those related to stress or trauma, PTSD, anxiety, depression, and substance abuse. Insurance is not a factor. You and I decide what thinking together is worth to you. Thinking together can include on-line and phone consultations. I don't know what you need to enjoy life more, but I'm willing to bet you do. We will figure that out, and make it happen. 
Forrest Seymour
Clinical Social Work/Therapist, LICSW Keene, NH 03431 (Online Only)
I have been a therapist for over 20 years and enjoy working with a wide variety of people and issues. I work with adults of all ages on anxiety, depression, family & relationship challenges, life changes, major transitions, trauma & PTSD, identity, and more. Together we can co-create spaces and experiences that meet your immediate and long-term needs, whether that be for insight, peace, justice, or healing. You are worth it. I have been a therapist for over 20 years and enjoy working with a wide variety of people and issues. I work with adults of all ages on anxiety, depression, family & relationship challenges, life changes, major transitions, trauma & PTSD, identity, and more. Together we can co-create spaces and experiences that meet your immediate and long-term needs, whether that be for insight, peace, justice, or healing. You are worth it. 
William Swinburne, PhD
Psychologist, PhD Verified I am a psychologist with over 30 years of clinical experience and a post-doc in Psychoanalytic psychotherapy. I work with both individuals and couples . My goals are to not only alleviate current suffering from acute distress, whether it be from depression, anxiety or other sources, but to also identify and address the causes of the emotional distress. I find it is helpful to understand your symptoms in the context of your entire life. If you feel this approach could be helpful to you, supply me a call. I am a psychologist with over 30 years of clinical experience and a post-doc in Psychoanalytic psychotherapy. I work with both individuals and couples . My goals are to not only alleviate current suffering from acute distress, whether it be from depression, anxiety or other sources, but to also identify and address the causes of the emotional distress. I find it is helpful to understand your symptoms in the context of your entire life. If you feel this approach could be helpful to you, supply me a call. 
Joshua Green
Not accepting new clients
If you are struggling with emotional pain that is confusing and/or seemingly uncontrollable and unpredictable, I may be a good fit for you. I frequently work with adults and college students who are struggling with anger, anxiety/panic, depression, relationship difficulties, sexual concerns, and distress related to family and life transitions. I also have significant experience working with individuals with histories of abuse, but I see people struggling with a wide variety of difficulties and concerns. I work with people of all genders, sexualities, and socioeconomic, racial, and cultural backgrounds. If you are struggling with emotional pain that is confusing and/or seemingly uncontrollable and unpredictable, I may be a good fit for you. I frequently work with adults and college students who are struggling with anger, anxiety/panic, depression, relationship difficulties, sexual concerns, and distress related to family and life transitions. I also have significant experience working with individuals with histories of abuse, but I see people struggling with a wide variety of difficulties and concerns. I work with people of all genders, sexualities, and socioeconomic, racial, and cultural backgrounds. Distinguished Sex Therapist Rhiannon Beauregard 
Alberto Soto
Psychologist, PhD Verified My areas of expertise are multicultural psychology and research related to the therapeutic relationship . I approach treatment from a relational-psychodynamic perspective that emphasizes the therapeutic relationship . During our work together, I seek to adapt therapy to each client, ensuring that every approach is tailored to your unique background and presenting concerns. My research background allows me to integrate various psychotherapy theories into treatment (CBT, interpersonal, mindfulness, multicultural, social justice approaches, etc.). My areas of expertise are multicultural psychology and research related to the therapeutic relationship . I approach treatment from a relational-psychodynamic perspective that emphasizes the therapeutic relationship . During our work together, I seek to adapt therapy to each client, ensuring that every approach is tailored to your unique background and presenting concerns. My research background allows me to integrate various psychotherapy theories into treatment (CBT, interpersonal, mindfulness, multicultural, social justice approaches, etc.). 
Ken Susskind, LICSW
Clinical Social Work/Therapist, MSW, LICSW Keene, NH 03431 (Online Only)
My specialties include anxiety and depression arising out of relationship issues- whether they be family-of-origin, current nuclear family, or partner / spouse . I have worked as a personal counselor since 1986 and as a licensed clinical social worker since 2004. I am a solution-focused therapist who listens deeply to my clients, then engages each in a true two-way conversation. Whether I'm seeing adults, older adolescents, or couples , I treat my clients respectfully. We focus on the issue at hand, work to understand the history and causes behind it, then together implement steps to bring change quickly. I don't draw out therapy. Most of my clients see me for six to eight weekly sessions, moving to less and less frequent sessions as their confidence and life satisfaction increase. My specialties include anxiety and depression arising out of relationship issues- whether they be family-of-origin, current nuclear family, or partner / spouse . I have worked as a personal counselor since 1986 and as a licensed clinical social worker since 2004. I am a solution-focused therapist who listens deeply to my clients, then engages each in a true two-way conversation. Whether I'm seeing adults, older adolescents, or couples , I treat my clients respectfully. We focus on the issue at hand, work to understand the history and causes behind it, then together implement steps to bring change quickly. I don't draw out therapy. Most of my clients see me for six to eight weekly sessions, moving to less and less frequent sessions as their confidence and life satisfaction increase. 
Keene Psychotherapy Services, Richard C. Donovan
Clinical Social Work/Therapist, MSW, ACSW, LICSW Verified Specialties include: dyadic work for couples , embodied breathwork, primal work, (deep feeling therapy) voice dialogue, energy work, gestalt somatic process work for trauma reprocessing, EFT (Emotionally focused treatment), TAT (Tapas Accupressure Technique), East/West Meditation, Bio-energetics, EMDR, and inner child work. I am a certified clinical traumatologist and psychotherapist. It is my hope to help you feel better. Whether its depression, fear worry, anxiety, stress, child problems, relationship concerns or family issues, we as a team will seek the solutions that will help you embrace the life you seek for yourself. Specialties include: dyadic work for couples , embodied breathwork, primal work, (deep feeling therapy) voice dialogue, energy work, gestalt somatic process work for trauma reprocessing, EFT (Emotionally focused treatment), TAT (Tapas Accupressure Technique), East/West Meditation, Bio-energetics, EMDR, and inner child work. I am a certified clinical traumatologist and psychotherapist. It is my hope to help you feel better. Whether its depression, fear worry, anxiety, stress, child problems, relationship concerns or family issues, we as a team will seek the solutions that will help you embrace the life you seek for yourself. 
Janet Morrison
Keene, NH 03431 (Online Only)
I offer a sex-positive, non-judgmental, and non-pathologizing coaching approach that will guide you to identify and move past your sexual/ relationship issues and empower you to attain your authentic sexuality/ relationship goals. When sexual issues are a concern, who do you turn to? It could be that you are suffering from a lack of intimacy in your relationship , an inability to experience pleasure or orgasm, or maybe you are wondering if your desires and fantasies are "normal." Have you sought out help only to be told "just live with it" or "sex isn't that important, anyway"? Or maybe your concerns have just been ignored. Do you want real solutions to your sexual concerns? I can help. I have extensive education and a passion for helping couples /individuals explore and regain pleasure. I offer a sex-positive, non-judgmental, and non-pathologizing coaching approach that will guide you to identify and move past your sexual/ relationship issues and empower you to attain your authentic sexuality/ relationship goals. When sexual issues are a concern, who do you turn to? It could be that you are suffering from a lack of intimacy in your relationship , an inability to experience pleasure or orgasm, or maybe you are wondering if your desires and fantasies are "normal." Have you sought out help only to be told "just live with it" or "sex isn't that important, anyway"? Or maybe your concerns have just been ignored. Do you want real solutions to your sexual concerns? I can help. I have extensive education and a passion for helping couples /individuals explore and regain pleasure. 
Jade Martin-Willis
Counselor, LCMHC, LMHC, R-DMT Keene, NH 03431 (Online Only)
I can help with but am not limited to helping people with grief, anxiety, depression, major life transitions, relationship issues, adjustment disorders, trauma related disorders and disordered eating patterns. I am a licensed clinician in New Hampshire and Massachusetts, and I am a Registered Dance/Movement Therapist. I have a person-centered and holistic approach. I enjoy blending traditional talk therapy, expressive arts, and movement therapy in my counseling sessions. Typically I begin therapy with traditional talk therapy and then at that point I am open to using any particular style depending on your preferred way of processing. Typically my clients are people who feel overwhelmed, have latest loss, need help improving self-esteem, need help improving relationships, and want realistic coping skills in their life. I can help with but am not limited to helping people with grief, anxiety, depression, major life transitions, relationship issues, adjustment disorders, trauma related disorders and disordered eating patterns. I am a licensed clinician in New Hampshire and Massachusetts, and I am a Registered Dance/Movement Therapist. I have a person-centered and holistic approach. I enjoy blending traditional talk therapy, expressive arts, and movement therapy in my counseling sessions. Typically I begin therapy with traditional talk therapy and then at that point I am open to using any particular style depending on your preferred way of processing. Typically my clients are people who feel overwhelmed, have latest loss, need help improving self-esteem, need help improving relationships, and want realistic coping skills in their life. 
Sarah Cushing
Clinical Social Work/Therapist, LICSW Verified Keene, NH 03431 (Online Only)
Not accepting new clients
In my 10 years of practice, I have found a true passion for working with women coping with anxiety, depression, women's health issues, relationship issues, and life transitions (such as parenthood, divorce, career changes, quarter and mid-life crisis, and retirement). **AS OF MARCH 2022, PRACTICE IS FULL AND WAIT LIST IS AT CAPACITY** Are you a strong, independent woman who feels stuck, dissatisfied, or unhappy in your life? Are you facing a women's health issue? Are you coping with a transition related to the end of a relationship , a shift in your career, a sudden or unexpected loss, or a quarter or mid-life crisis? If the answer to any of these question is "YES" then I can help! In my 10 years of practice, I have found a true passion for working with women coping with anxiety, depression, women's health issues, relationship issues, and life transitions (such as parenthood, divorce, career changes, quarter and mid-life crisis, and retirement). **AS OF MARCH 2022, PRACTICE IS FULL AND WAIT LIST IS AT CAPACITY** Are you a strong, independent woman who feels stuck, dissatisfied, or unhappy in your life? Are you facing a women's health issue? Are you coping with a transition related to the end of a relationship , a shift in your career, a sudden or unexpected loss, or a quarter or mid-life crisis? If the answer to any of these question is "YES" then I can help! 
Roger L Peterson
Psychologist, PhD, ABPP Verified Keene, NH 03431 (Online Only)
Roger L. Peterson is a Clinical Psychologist practicing teleheaith in New Hampshire. He works from a cognitive interpersonal orientation and has been influenced by evidence based treatments. He also does supervision, consultation, and teaching. Dr. Peterson’s areas of focus are: Couples , people with interpersonal concerns or health issues, and older people. He takes most insurances but not Medicare. The practice is HIPAA compliant and is not an emergency mental health service. Roger L. Peterson received his doctorate in clinical psychology from Purdue University. Roger L. Peterson is a Clinical Psychologist practicing teleheaith in New Hampshire. He works from a cognitive interpersonal orientation and has been influenced by evidence based treatments. He also does supervision, consultation, and teaching. Dr. Peterson’s areas of focus are: Couples , people with interpersonal concerns or health issues, and older people. He takes most insurances but not Medicare. The practice is HIPAA compliant and is not an emergency mental health service. Roger L. Peterson received his doctorate in clinical psychology from Purdue University. Antioch Couple and Family Therapy Institute
Marriage & Family Therapist, PhD, LMFT Verified Sometimes we find ourselves struggling, unsure of how to make things better and most of us have experienced challenges within an important relationship . It’s important to know that at times like those, you are not alone. The Couple and Family Therapy Institute (CFTI) is here to help. Sometimes we find ourselves struggling, unsure of how to make things better and most of us have experienced challenges within an important relationship . It’s important to know that at times like those, you are not alone. The Couple and Family Therapy Institute (CFTI) is here to help. 
Kate Robertson
Counselor, LCMHC, CYT Verified Keene, NH 03431 (Online Only)
For over 14 years, I have worked with clients facing challenges such as addiction, anxiety, depression, trauma and difficult life changes. Together, we connect with their inner resources of self-compassion, wisdom and courage to uncover their authentic selves. When we cultivate a mindset of awareness and understanding, rather than judgment, we create the space to heal. Life experiences become a vehicle for learning and embracing our strength and resilience, as well as deepening our relationship with self and others. We strengthen our ability to live fully, with curiosity and openness to our journey. For over 14 years, I have worked with clients facing challenges such as addiction, anxiety, depression, trauma and difficult life changes. Together, we connect with their inner resources of self-compassion, wisdom and courage to uncover their authentic selves. When we cultivate a mindset of awareness and understanding, rather than judgment, we create the space to heal. Life experiences become a vehicle for learning and embracing our strength and resilience, as well as deepening our relationship with self and others. We strengthen our ability to live fully, with curiosity and openness to our journey. See more therapy options for Keene Couples Counseling Therapists
Does couples counseling work?Research shows that couples counseling is effective; it can reduce relationship distress and Boost emotional awareness, communication, empathy, intimacy, and overall relationship satisfaction. For example, emotionally focused therapy was found to be effective for about 75 percent of couples, and those benefits lasted at least two years.
When should a couple seek counseling?Couples can benefit from counseling if they consistently struggle in their relationship. They may have lost the ability to communicate effectively, become entrenched in harmful patterns, begun to fight more than usual, broken the other’s trust, suffered from a mental or physical illness, or faced any number of other challenges. Many therapists offer free consultations, so if a couple isn’t sure whether therapy is necessary, asking directly can provide clarity. How can I get my partner to go to couples therapy?The decision to seek couples therapy is often driven by one partner, who then convinces the other to participate. When discussing the idea, the initiator should be direct and assertive. They can state the problems they see and explain how the relationship would benefit from therapy. In more serious cases, they can also explain how their relationship may suffer without making any changes or seeking professional help. How does a couple prepare for couples counseling?The anticipation of beginning couples counseling can be difficult—sometimes more difficult than the first session itself. Taking a few moments to reflect can allay those concerns: What are the current challenges? When and how did they begin? What do they want the relationship to look like in the future? Reflecting on these questions can help individuals or couples articulate their goals. Of course, the therapist will also ask questions and guide couples through the process. | ||||||||||||||||||||||
HIO-201 exam plan | HIO-201 student | HIO-201 tricks | HIO-201 course outline | HIO-201 student | HIO-201 plan | HIO-201 availability | HIO-201 guide | HIO-201 download | HIO-201 pdf | | ||||||||||||||||||||||
Killexams exam Simulator Killexams Questions and Answers Killexams Exams List Search Exams |